You are here: OxNotes Home › IGCSE Chemistry › Atoms
Simple explanation of an Atom
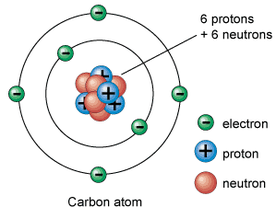
Atoms are made up of sub-atomic (smaller than an atom) particles called protons, neutrons and electrons. The protons and neutrons form the core of the atom, this is called the nucleus. The nucleus is orbited by electrons. Atoms are the smallest part of an element that can exist; the word atom derives from the Ancient Greek word “atomos” (a meaning “not” and tomos meaning “cut”) that means incapable of being divided (indivisible). Everything is made up of atoms.
Proton, Neutron and Electrons
The properties of atoms depend on the arrangement of the three sub-atomic particles: protons, neutrons and electrons. Keep in mind that because they are so small, there is no real unit for the mass or charge of these sub-atomic particles. The term “atomic mass unit” and “elementary charge (e)” are used in proportion to each of the other sub-atomic particles.
Sub-atomic particles are too small to be measured accurately by the normal scale. So instead we weigh them in proportion to one another. Say a proton weighs 1. In proportion to a proton, a neutron also weighs 1 and an electron weighs 1/1836 . Give these the unit of “atomic mass unit”, and the sub-atomic particles now have recordable masses. Same method applies for their charges.
The number of protons in an atom determines what element it is, e.g if an atom has 8 protons in its nucleus it will always be Oxygen, even if the atom with 8 protons is bonded to something else and is part of a chemical it is still Oxygen. The proton number is equal to the atomic number, the atomic number is written on the Periodic Table. The electron number can always change as the atom exchanges or gives electrons to other elements but the proton number is quite stable. A change in the number of neutrons would not affect what element the atom is. If the proton number (or atomic number) changes, the element also changes.
Sub-atomic particles are too small to be measured accurately by the normal scale. So instead we weigh them in proportion to one another. Say a proton weighs 1. In proportion to a proton, a neutron also weighs 1 and an electron weighs 1/1836 . Give these the unit of “atomic mass unit”, and the sub-atomic particles now have recordable masses. Same method applies for their charges.
- Proton - Has a positive charge ( +1 ). It weighs one atomic mass unit and is found in the nucleus.
- Neutron - Has a neutral charge ( 0 ). It weighs one atomic mass unit and is also found in the nucleus.
- Electron - Has a negative charge (-1) and almost no mass at all. It weighs 1/1836 of one atomic unit and orbits around the nucleus.
The number of protons in an atom determines what element it is, e.g if an atom has 8 protons in its nucleus it will always be Oxygen, even if the atom with 8 protons is bonded to something else and is part of a chemical it is still Oxygen. The proton number is equal to the atomic number, the atomic number is written on the Periodic Table. The electron number can always change as the atom exchanges or gives electrons to other elements but the proton number is quite stable. A change in the number of neutrons would not affect what element the atom is. If the proton number (or atomic number) changes, the element also changes.
- The number of protons in an atom determines what element it is. The number of protons is represented by the atomic number on the Periodic Table, thus showing that the atomic number is equal to the proton number. An element in its original state will have the same number of electrons as it does protons (therefore cancelling out the charges to make the atom neutral/uncharged).
- The number of electrons changes frequently due to various reactions taking place. An atom may receive, give or share an electron with another atom, therefore varying the number of electrons it may have at any given time. If an atom has an unequal number of electrons versus protons, it will obtain a charge. These charged atoms are called ions.
- The number of neutrons in an atom should stay the same. However, sometimes an atom may have more or less neutrons than the elemental atom has in its original form. This then changes the atomic mass number (shown on the periodic table) and the atom is then called an isotope. A varying number of neutrons does not affect the chemical qualities of the atom. It will still react in the same way because the number of neutrons does not affect the overall charge, due to their neutral charge of 0.
Compounds and Molecules
A molecule is two or more atoms bonded together.
When two different atoms bond, e.g. Hydrogen and Oxygen (Water), it is called a compound.
A molecule consists of two or more atoms chemically bonded together, these atoms can be the same type or different atoms. A molecule of two oxygen atoms, O2 (Oxygen), is a molecule but is not a compound.
A compound is usually a molecule as well. A compound must have atoms of different elements bonded together, eg. Two hydrogen atoms and one oxygen atom bonded together, which makes the compound -CHECK THE 2!! (water).
When two different atoms bond, e.g. Hydrogen and Oxygen (Water), it is called a compound.
A molecule consists of two or more atoms chemically bonded together, these atoms can be the same type or different atoms. A molecule of two oxygen atoms, O2 (Oxygen), is a molecule but is not a compound.
A compound is usually a molecule as well. A compound must have atoms of different elements bonded together, eg. Two hydrogen atoms and one oxygen atom bonded together, which makes the compound -CHECK THE 2!! (water).
Experiments to investigate the small size of particles and their movement
Dilution of coloured solutions: Coming Soon.
Diffusion experiments: Coming Soon.
Diffusion experiments: Coming Soon.