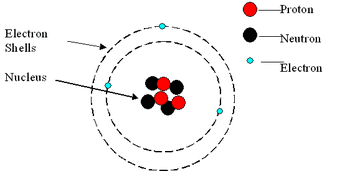
Atoms: Central Nucleus, Protons, Neutrons and Electrons
The centre of an atom is called the nucleus, composed of protons and neutrons, surrounded by electrons which orbit in a series of energy levels which are called shells. Each electron shell is able to hold a particular number of electrons. The first shell outside the nucleus holds up two electrons, the second and third shell both hold a maximum of eight.
The masses of protons and neutrons are pretty equal, whereas the mass of the electron is almost negligible, most of the mass of an atom is in the nucleus. Electrons are much smaller than both protons and neutrons.