- The three states of matter
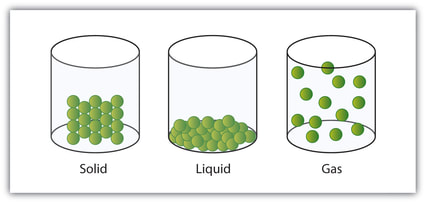
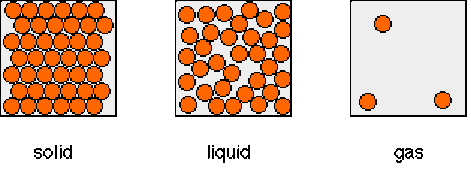
The arrangement, movement and energy of the particles in each of the three states of matter: solid, liquid and gas.
Solid: Particles in a solid are still and have no energy, the particles aren't moving. All of the particles in a solid are touching and are compact.
Liquid: Particles in a liquid are moving and can flow to fill and fit a space, the particles have some energy. Like solids, all of the particles are touching.
Gas: Particles in a gas move freely and have lots of energy (a high energy level). The particles are far apart aren't touching.
Liquid: Particles in a liquid are moving and can flow to fill and fit a space, the particles have some energy. Like solids, all of the particles are touching.
Gas: Particles in a gas move freely and have lots of energy (a high energy level). The particles are far apart aren't touching.
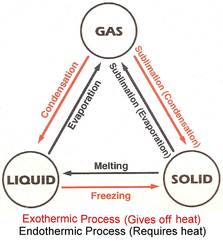
Changing states
The conversions of solids, liquids and gases are achieved through the following processes:
A small number of substances have the ability to change directly from Gas to Solid or Solid to Gas, without involving any liquid state on the way. This process is called sublimation:
|

An example is carbon dioxide. There is no such thing as liquid carbon dioxide. It turns straight from a solid to a gas at -78 °C. Solid carbon dioxide is known as dry ice.
In the image on the left, you can see that solid carbon dioxide turns straight to a gas. The white gas appears because the carbon dioxide produced is so cold that is causes water vapour in the air to condense. Carbon dioxide gas itself is invisible.
In the image on the left, you can see that solid carbon dioxide turns straight to a gas. The white gas appears because the carbon dioxide produced is so cold that is causes water vapour in the air to condense. Carbon dioxide gas itself is invisible.