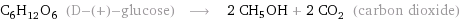Simple Explanation of Ethanol
Ethanol is a chemical compound. When people refer to it, they often name it simply as alcohol. The familiar alcohol in drinks is C2H5OH or C2H60 (CH3-CH2-OH), and should properly be called ethanol.
CH3–CH2–OH indicates the carbon of a methyl group (CH3–) is attached to the carbon of a methylene group (–CH2–), which is attached to the oxygen of a hydroxyl group (–OH).
Topics covered on this page (Ethanol):
Manufacture of ethanol
Fermentation of sugar
Hydration of ethene
Manufacture of ethanol
Fermentation of sugar
Hydration of ethene
Manufacture of ethanol
Ethanol is manufactured by two different processes:
- Fermentation of sugar
- Hydration of ethene
Fermentation of sugar
- Dissolve sugar/starch in water and add yeast to this solution.
- Leave the mixture to ferment in warm conditions (25-40°C) for several days in the absence of air (anaerobic conditions, which means without oxygen present).
- Enzymes (biological catalysts) in the yeast convert the sugar into ethanol and carbon dioxide. This process is called fermentation.
- Filter off the excess yeast to obtain a dilute solution of ethanol.
Whatever the starting point, sugar or starch, the enzymes in the yeast produce glucose, C6H12O6. The enzymes in yeast then convert the glucose into ethanol:
Hydration of ethene
Ethanol can also be made by reacting ethene with steam. This process is called hydration.
- Mixture of ethene and steam is passed over a phosphoric acid catalyst at a temperature of 300°C and 60-70 atmospheres of pressure.
- The ethanol is condensed as a liquid.
- The ethene required for this reaction is obtained from crude oil.