OxNotes > GCSE/IGCSE Revision > IGCSE Chemistry > Industrial manufacture of Ammonia / Haber Process
The manufacturing of ammonia
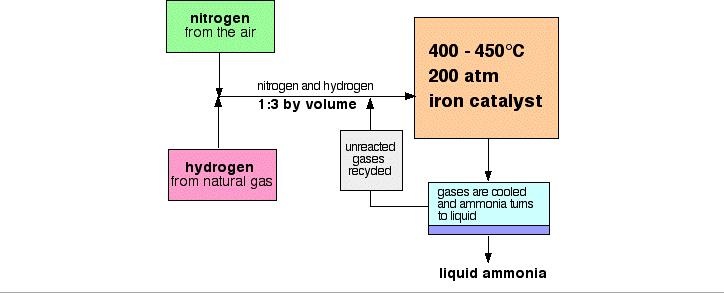
Nitrogen (from air), and hydrogen (from natural gas (methane - CH4) or the cracking of hydrocarbons), are reacted to make ammonia.
Manufacture of ammonia by the Haber Process
The essential conditions:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Decreasing the temperature would increase the yield (the reaction prefers a lower temperature which is why it’s releasing heat), however, it is still done at a fairly high temperature to speed up the rate of reaction and create ammonia faster. The reaction would be slower at low temperatures. It would be useless to have a low temperature and achieve a high yield of ammonia if it takes too long to create the product.
The catalyst does not affect the amount of product made. The yield of ammonia stays the same, but is made faster as the catalyst speeds up the reaction by lowering the activation energy needed for the reaction.
Increasing the pressure would favour the forwards reaction, which is desired as it means more ammonia is made. This increase in forward reaction is due to their being less moles of gas on the right side (in the balanced equation above, there are 4 moles of gas on the reactants side (left) and 2 moles of gas (ammonia) on the right hand side (products), so according to Le Chatelier's principle where you try to remove the change, if you increase pressure, the equilibrium would move to the right hand side to decrease pressure. And the products (right side) have less pressure because there are less molecules on that side so it is favoured (2 moles compare to 4 on the left).
Rate considerations (pressure)
Increasing the pressure brings the molecules closer together. In this particular instance, it will increase their chances of hitting and sticking to the surface of the catalyst where they can react. The higher the pressure the better in terms of the rate of a gas reaction because more of the product is created.
Economic considerations
The compromise
200 atmospheres is a compromise pressure chosen on economic grounds. If the pressure used is too high, the cost of generating it exceeds the extra profit made from the extra ammonia produced.
- A temperature of about 450°C
- A pressure of about 200 atmospheres
- An iron catalyst
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Decreasing the temperature would increase the yield (the reaction prefers a lower temperature which is why it’s releasing heat), however, it is still done at a fairly high temperature to speed up the rate of reaction and create ammonia faster. The reaction would be slower at low temperatures. It would be useless to have a low temperature and achieve a high yield of ammonia if it takes too long to create the product.
The catalyst does not affect the amount of product made. The yield of ammonia stays the same, but is made faster as the catalyst speeds up the reaction by lowering the activation energy needed for the reaction.
Increasing the pressure would favour the forwards reaction, which is desired as it means more ammonia is made. This increase in forward reaction is due to their being less moles of gas on the right side (in the balanced equation above, there are 4 moles of gas on the reactants side (left) and 2 moles of gas (ammonia) on the right hand side (products), so according to Le Chatelier's principle where you try to remove the change, if you increase pressure, the equilibrium would move to the right hand side to decrease pressure. And the products (right side) have less pressure because there are less molecules on that side so it is favoured (2 moles compare to 4 on the left).
Rate considerations (pressure)
Increasing the pressure brings the molecules closer together. In this particular instance, it will increase their chances of hitting and sticking to the surface of the catalyst where they can react. The higher the pressure the better in terms of the rate of a gas reaction because more of the product is created.
Economic considerations
- Very high pressures are very expensive to produce on two counts.
- You have to build extremely strong pipes and containment vessels to withstand the very high pressure. That increases capital costs.
- High pressures cost a lot to maintain, resulting in high running costs of the manufacturing plant.
The compromise
200 atmospheres is a compromise pressure chosen on economic grounds. If the pressure used is too high, the cost of generating it exceeds the extra profit made from the extra ammonia produced.
Ammonia: Cooling of the reaction mixture
The cooling of the reaction mixture liquefies the ammonia produced and allows the unused hydrogen and nitrogen to be recirculated.
Separating the ammonia
When the gases leave the reactor they are hot and under high pressure. Ammonia is easily liquefied under pressure as long as it isn't too hot, thus the temperature of the mixture is lowered. The nitrogen and hydrogen remain as gases even under these high pressures and are recycled.
Recycling
At each pass of the gases through the reactor, only about 15% of the nitrogen and hydrogen converts to ammonia. By continual recycling of the unreacted nitrogen and hydrogen, the overall conversion is about 98%.
When the gases leave the reactor they are hot and under high pressure. Ammonia is easily liquefied under pressure as long as it isn't too hot, thus the temperature of the mixture is lowered. The nitrogen and hydrogen remain as gases even under these high pressures and are recycled.
Recycling
At each pass of the gases through the reactor, only about 15% of the nitrogen and hydrogen converts to ammonia. By continual recycling of the unreacted nitrogen and hydrogen, the overall conversion is about 98%.