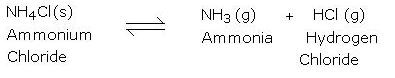OxNotes > Chemistry IGCSE Revision > Equilibria / Reversible, Dynamic Equilibriums Reactions
Simple explanation of Reversible Reactions
Some reactions are reversible (they can react both ways, forwards and backwards), the reactants can make the products and the products can react again to make the reactants. Instead of a one way arrow the right (--->), reactions that are reversible are indicated by the symbol “⇌” in equations. Examples of reversible reactions are described below.
Simple explanation of Dynamic Equilibriums
The concept of dynamic equilibriums is when a reversible reaction is happening both ways at the same time, at the same rate. Because the reaction is happening at the same rate both ways, it does not change.
Examples of reversible reactions
You need to be able to describe reversible reactions such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride:
- Dehydration of hydrated copper(II) sulfate
If you remove water from hydrated copper sulphate, copper sulphate (anhydrous copper sulphate) is formed.
- Effect of heat on ammonium chloride
Hydrogen chloride and ammonia can be reacted to form ammonium chloride.
Effects of changing pressure/ temperature on the equilibrium position in reversible reactions
By changing the pressure and temperature, the position of the dynamic equilibrium (the conditions where the reaction is equal and not changing) is shifted. If you move the equilibrium, you change the rate of reaction.
Increase in pressure = equilibrium shifts to the side with the most molecules.
Increase in temperature = will be more products that are produced by an endothermic reaction. This is due to the reaction trying to use up the extra heat, it does so by putting the energy into making bonds. Making bonds requires energy so it takes in energy (endothermic reaction).
- If the equilibrium shifts/moves to the right, you have more products (left side of the equation). This means the reactants are reacting faster.
- If the equilibrium shifts to the left, you have more reactants (right side of the equation). This means the products are reacting faster.
Increase in pressure = equilibrium shifts to the side with the most molecules.
Increase in temperature = will be more products that are produced by an endothermic reaction. This is due to the reaction trying to use up the extra heat, it does so by putting the energy into making bonds. Making bonds requires energy so it takes in energy (endothermic reaction).