1.16 calculate relative formula masses (Mr) from relative atomic masses (Ar)
Relative formula mass is the sum of mass of all atoms in a formula, it is given the symbol, Mr.
Relative atomic mass is the average mass of all the isotopes of an element. It is given the symbol, Ar.
To calculate the Mr of a substance, all you have to do is add up the relative atomic masses of all the atoms present in the formula.
Water- H₂0
Atoms present:(2 x H) + (1 x O)
Mr= (2 x 1) + 16= 18
(There is 1 electron per hydrogen atom, and 2 hydrogen atoms, so that is 2 electrons. There is also 1 oxygen atom, which has 16 electrons, 2 + 16= 18)
Examples:
Relative atomic mass is the average mass of all the isotopes of an element. It is given the symbol, Ar.
To calculate the Mr of a substance, all you have to do is add up the relative atomic masses of all the atoms present in the formula.
Water- H₂0
Atoms present:(2 x H) + (1 x O)
Mr= (2 x 1) + 16= 18
(There is 1 electron per hydrogen atom, and 2 hydrogen atoms, so that is 2 electrons. There is also 1 oxygen atom, which has 16 electrons, 2 + 16= 18)
Examples:
- Carbon Dioxide (CO2) - Has 1 carbon and 2 oxygens, C=12 O=16, Ar = 12 + (16 x 2), Mr= 44
- Oxygen (O2), 2 oxygens, O=16, Ar= 16 x 2, Mr = 32
- Aluminium Oxide (Al2O3), 2 aluminium, 3 oxygens, Al= 27 O=16, Ar= (27 x 2) + (16 x 3), Mr = 102
- Sodium Sulphate (Na2SO4), 2 Sodiums 1 sulphur and 4 oxygens, Na= 23 S= 32 O=16, Ar= (23 x2) + 32 + (16 x 4), Mr= 142
1.17 understand the use of the term mole to represent the amount of substance
1 mole = 602,000,000,000,000,000,000,000 = 6.02x10^ 23 (^ = to the power of)
Moles are used for a similar reason to why light years are used in Physics, the numbers are so huge.
The mole is a measure of the amount of substance. 1 mol is the amount of substance that contains 6 x 10 ^23 particles of the substance.
Having 12 doughnuts is similar to having a carbon. In the same way that you can have a dozen doughnuts, you can have a mole of carbon.
Having a mole of something means having 6.022x10 ^23 of it.
The mass of one mole of atoms is easily calculated, it is the relative atomic mass expressed in grams.
(The following example is used for 1.19 as well)
Eg. In 23g of Na, there are 6.022x10 ^23 (1 Mole = 23g of Na) (23g/m) - Atomic mass of carbon is 23
In 40g of Ca, there are 6.022x10 ^23 (40g/m) - Atomic mass of calcium is 40
Moles are used for a similar reason to why light years are used in Physics, the numbers are so huge.
The mole is a measure of the amount of substance. 1 mol is the amount of substance that contains 6 x 10 ^23 particles of the substance.
Having 12 doughnuts is similar to having a carbon. In the same way that you can have a dozen doughnuts, you can have a mole of carbon.
Having a mole of something means having 6.022x10 ^23 of it.
The mass of one mole of atoms is easily calculated, it is the relative atomic mass expressed in grams.
(The following example is used for 1.19 as well)
Eg. In 23g of Na, there are 6.022x10 ^23 (1 Mole = 23g of Na) (23g/m) - Atomic mass of carbon is 23
In 40g of Ca, there are 6.022x10 ^23 (40g/m) - Atomic mass of calcium is 40
1.18 understand the term mole as the Avogadro number of particles (atoms, molecules, formulae, ions or electrons) in a substance
The mole is a measure of the amount of substance. 1 mol is the amount of substance that contains 6 x 10 ^23 particles of the substance.
This is known as the Avogadro Number.
(A mole is Avogadro's number because if you have 1 mole of an element its weight in grams will be its atomic mass.)
This is known as the Avogadro Number.
(A mole is Avogadro's number because if you have 1 mole of an element its weight in grams will be its atomic mass.)
1.19 carry out mole calculations using relative atomic mass (Ar) and relative formula mass (Mr)
1.20 understand the term molar volume of a gas and use its values (24 dm3 and 24,000 cm3) at room temperature and pressure (rtp) in calculations.
The mass of one mole of atoms is easily calculated, it is the relative atomic mass expressed in grams.
(The examples below have been used in 1.17 as well)
³Eg. In 23g of Na, there are 6.022x10 ^23 (1 Mole = 23g of Na) (23g/m) - Atomic mass of carbon is 23
In 40g of Ca, there are 6.022x10 ^23 (40g/m) - Atomic mass of calcium is 40
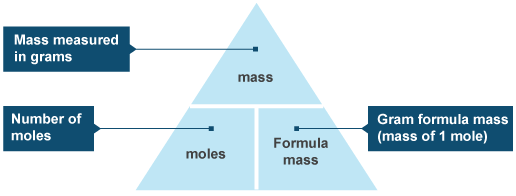
The 'Mole Triangle' may help, with Mass (g) on top, Moles (mol) bottom left, and Mr/Ar bottom right
(The examples below have been used in 1.17 as well)
³Eg. In 23g of Na, there are 6.022x10 ^23 (1 Mole = 23g of Na) (23g/m) - Atomic mass of carbon is 23
In 40g of Ca, there are 6.022x10 ^23 (40g/m) - Atomic mass of calcium is 40
The 'Mole Triangle' may help, with Mass (g) on top, Moles (mol) bottom left, and Mr/Ar bottom right
At normal temperature and pressure, one mole of any gas will occupy 24000 cm³; also known as 24dm³. This is called the molar volume of gas.
A good way to do calculations with this information is by using the triangle in 1.19 (above)
e.g. Calculate the amount of carbon dioxide gas collected if you collect 144cm3 (in moles).
So we do mass over formula mass, 144 / 24000
= 0.006 Mol
If we had 3 mols of H, the volume of the gas would be 72dm³ (3 x 24)
A good way to do calculations with this information is by using the triangle in 1.19 (above)
e.g. Calculate the amount of carbon dioxide gas collected if you collect 144cm3 (in moles).
So we do mass over formula mass, 144 / 24000
= 0.006 Mol
If we had 3 mols of H, the volume of the gas would be 72dm³ (3 x 24)